Continuous Glucose Moniot Adverse Events Freestyle
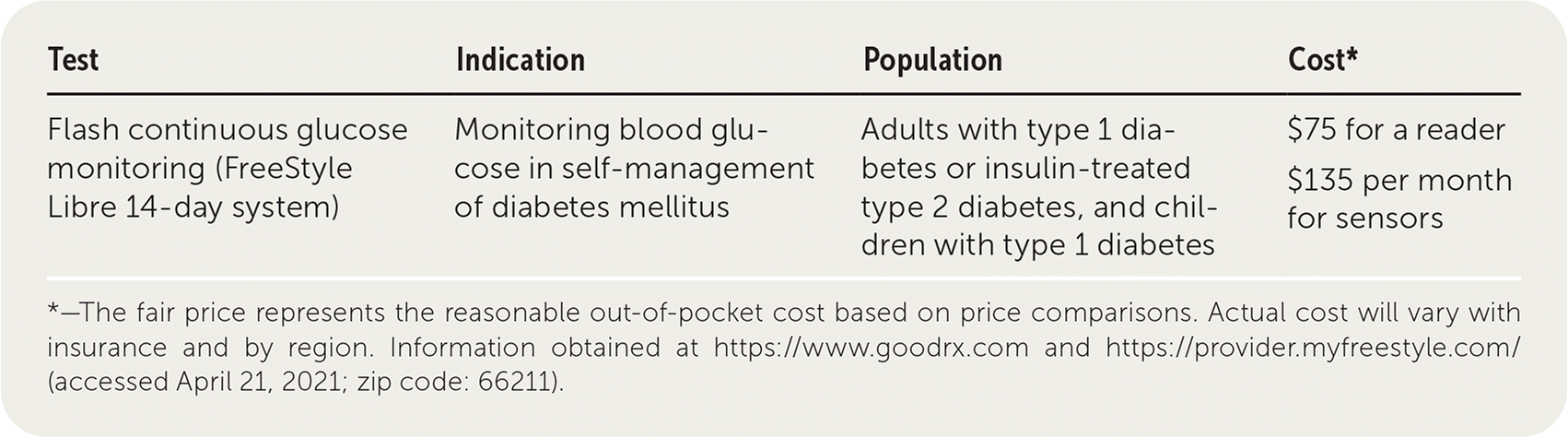
Continuous glucose monitors (CGMs) are used for the self-management of diabetes mellitus and have a subcutaneously inserted sensor that measures glucose in the interstitial fluid and transmits the result to a receiver. The FreeStyle Libre 14-day system is an intermittently scanned or "flash" CGM that was approved by the U.S. Food and Drug Administration in 2017. It displays glucose values when the sensor is scanned with the receiver. The sensor is placed on the posterior upper arm, lasts 14 days, and is factory calibrated. FreeStyle Libre displays eight-hour glucose trends but does not have any alarms.1,2 Real-time CGMs measure glucose every one to five minutes and issue alarms when glucose values are too high or low. Some brands may need to be calibrated by self-monitoring of capillary blood glucose (SMBG), which uses a finger stick and test strips.1,2
Accuracy
FreeStyle Libre is accurate in adults and children (mean absolute relative difference = 11.4% and 13.9%, respectively), with capillary blood glucose as the reference standard.3,4 Its accuracy is stable throughout the 14-day lifespan of the sensor but is lower during hypoglycemia and exercise and after a glucose load.3,5,6
Benefit
A systematic review and meta-analysis of 15 randomized controlled trials (RCTs) evaluated the impact of CGMs (including real-time and flash CGMs) on glycemic control compared with usual care using SMBG (n = 2,477).7 It included three studies of FreeStyle Libre in adults; two of the studies evaluated adults with type 1 diabetes and one study evaluated adults with insulin-treated type 2 diabetes. In general, CGMs produced a pooled A1C reduction of −0.17% (95% CI, −0.29% to −0.06%), greater time in euglycemic range (70.74 minutes per day; 95% CI, 46.73 to 94.76), less time in hyperglycemia (−30.26 minutes per day; 95% CI, −58.15 to −2.38), and less time in hypoglycemia (−27.16 minutes per day; 95% CI, −42.08 to −12.25). A subanalysis of the FreeStyle Libre trials (n = 626) found similar effects on time in euglycemic range, hyperglycemia, and hypoglycemia, but no statistically significant A1C reduction.
A meta-analysis of 12 studies (three RCTs, five prospective cohorts, three retrospective cohorts, one not reported; n = 2,173) evaluating the use of FreeStyle Libre in adults and children with type 1 diabetes or insulin-treated type 2 diabetes showed that the use of FreeStyle Libre reduced A1C levels by −0.26% (95% CI, −0.43% to −0.09%) compared with baseline. Compared with SMBG, however, A1C reduction was no longer significant.8
ADULTS WITH TYPE 1 DIABETES
An RCT of adults with well-controlled type 1 diabetes (n = 241) showed no A1C reduction in patients using FreeStyle Libre compared with SMBG.9 FreeStyle Libre was associated with increased time in euglycemic range (one hour per day), decreased hypoglycemic events (−0.45 events per day), decreased time in hypoglycemia (−1.24 hours per day), and decreased time in hyperglycemia (−0.37 hours per day).9
A prospective cohort study of FreeStyle Libre found a decrease in the number of people admitted to the emergency department or hospital for hypoglycemia or diabetic ketoacidosis (from 3.3% to 2.2%), number of people with severe hypoglycemic episodes requiring third-party help (14.6% to 7.8%), number of people with hypoglycemic comas (2.7% to 1.1%), and diabetes-related absenteeism (5.8% to 2.9%) at 12 months compared with baseline.10
Although both studies found statistically significant improvements in treatment satisfaction (Diabetes Treatment Satisfaction Questionnaire score) and perceived frequency of hyperglycemia compared with SMBG, there were no improvements in quality of life, Diabetes Distress Scale score, or hypoglycemia fear scores.9,10
CHILDREN WITH TYPE 1 DIABETES
A meta-analysis of 10 observational studies (n = 908) showed an A1C reduction of −0.29% (95% CI, −0.47% to −0.10%) from baseline in children with type 1 diabetes using FreeStyle Libre.11 In an RCT comparing FreeStyle Libre with SMBG (n = 51), there was no difference in time in euglycemic range, hypoglycemic time or events, or hyperglycemic time or events.12 A prospective cohort study of FreeStyle Libre found a significant decrease in the proportion of patients with severe hypoglycemic events at 12 months (6.8% to 3.2%) compared with baseline, whereas there was no significant decrease in SMBG users.13
constantanium1957.blogspot.com
Source: https://www.aafp.org/pubs/afp/issues/2021/0601/p688.html
0 Response to "Continuous Glucose Moniot Adverse Events Freestyle"
Post a Comment